NORA520
A Product That Is More Convenient and Accessible for Patients
NORA520 is an oral prodrug which is hydrolyzed to Brexanolone (Allopregnanolone), the same active ingredient in the only FDA-approved drug specifically indicated for PPD. NORA520 provides an alternative convenient oral treatment to PPD patients and it potentially can become the first line therapy.
Mechanism of action
Allopregnanolone, a metabolite of progesterone, is an endogenous neuroactive steroid. Lower level of allopregnanolone is associated to postpartum depression. Allopregnanolone is one of the most potent extrasynaptic GABAA receptor modulators, and GABAergic dysfunction is closely related to the etiology of PPD.
The active moiety of NORA520, which is structurally identical to allopregnanolone, binds to extrasynaptic GABAA receptor and increases tonic GABAergic current.
Source:
- Hellgren C, Åkerud H, Skalkidou A, Bäckström T, Sundström-Poromaa I. Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology. 2014;69(3):147-53;
- Osborne LM, Gispen F, Sanyal A, Yenokyan G, Meilman S, Payne JL. Lower allopregnanolone during pregnancy predicts postpartum depression: An exploratory study. Psychoneuroendocrinology. 2017 May;79:116-121;
- Meltzer-Brody S, Kanes SJ. Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiol Stress. 2020 Feb 3;12:100212.
NORA520 Advantage
NORA520 has various advantages over the FDA-approved drug specifically indicated for PPD and current standard of care and allows for a much-improved patient experience. NORA520 is manufactured as an oral tablet, allowing for potential outpatient administration. The design of the prodrug NORA520 can overcome the difficulties and inconvenience of the current 60-hour IV infusion treatment regimen of the FDA-approved drug specifically indicated for PPD; at the same time remains the advantage of rapid onset of action, short treatment duration and same efficacy durability.
Oral administration
MOA leads to rapid onset of action
Brief treatment duration
Potential outpatient administration
Avoid AEs caused by infusion pump malfunction
Disease Indications:
Postpartum Depression Overview
Postpartum Blues
(“Baby Blues”)
- Occur in 50-80% of new mothers
- Symptom length <2 weeks
- Do not impair maternal function
Postpartum Depression
- Occur in 10-20% of all women
- Symptom length could be several months to one year
- Symptoms occur almost daily and may result in functional impairment
Postpartum Psychosis
- Occur in 0.1-0.2% of births
- Symptom are temporary but must be treated
- Mental health emergency putting both mother and child at risk
Target Population of PPD:
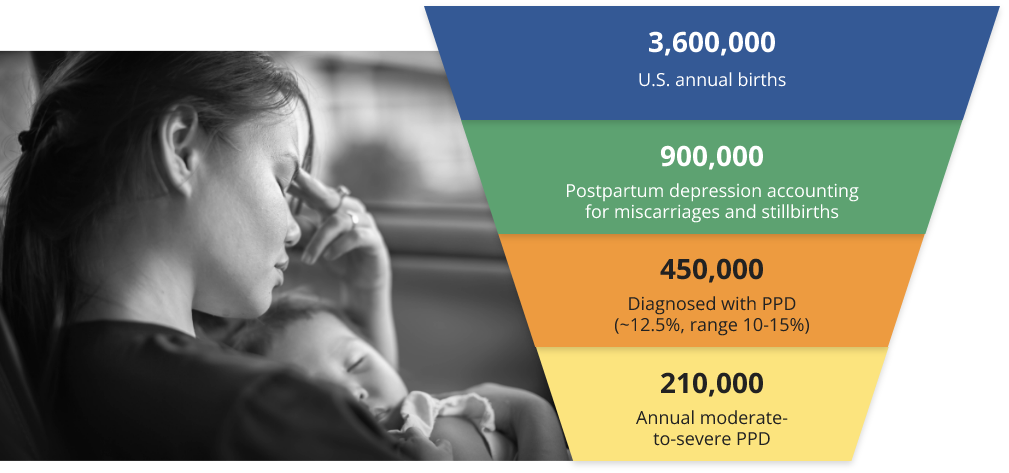
Postpartum depression (PPD) affects some women after childbirth and can cause considerable suffering, not only for mothers, but also for their close family members. It has been reported that 121 million women are affected globally. In the US, more than 900,000 women experience the “baby blues” each year, which includes still births and miscarriages, and 450,000 mothers are diagnosed with postpartum depression.
Source:
- CDC.gov; UpToDate, accessed Jan. 2022;
- Langdon, K. Postpartum depression statistics. 2022, retrieved from postpartumdepression.org;
- Postpartum Depression: Action Towards Causes and Treatment (PACT) Consortium. Lancet Psychiatry. 2015. 2(1):59-67;
- Shorey, S. et al. J PsychiatrRes. 2018. 104:235-248;
- Shidhaye PR and Giri PA. Maternal Depression: A Hidden Burden in Developing Countries. Ann Med Health Sci Res. 2014 Jul-Aug; 4(4): 463–465.
NORA520 Trials
NORA520-PT-US-1a is a Phase 2 clinical trial to test the study drug, NORA520, as a possible treatment for severe postpartum depression (PPD).
- Further details of NORA520 trials: www.clinicaltrials.gov




